
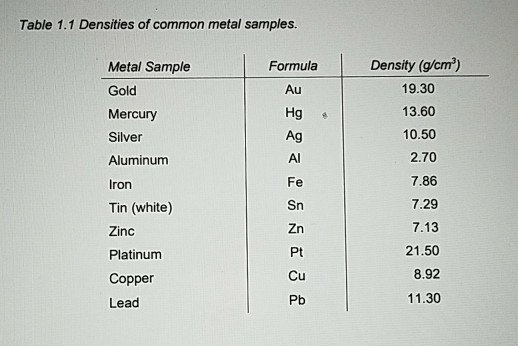
The symbol for Osmium is Os and its density g/cm 3 is 22.59.

The symbol for Niobium is Nb and its density g/cm 3 is 8.57.The symbol for Nickel is Ni and its density g/cm 3 is 8.90.The symbol for Neptunium is Np and its density g/cm 3 is 20.2.The symbol for Neodymium is Nd and its density g/cm 3 is 7.01.The symbol for Molybdenum is Mo and its density g/cm 3 is 10.2.The symbol for Mercury is Hg and its density g/cm 3 is 13.53.The symbol for Manganese is Mn and its density g/cm 3 is 7.3.The symbol of Magnesium is Mg and its density g/cm 3 is 1.74.The symbol for Lithium is Li and its density g/cm 3 is 0.53.The symbol for Lead is Pb and its density g/cm 3 is 11.3.The symbol for Lanthanum is La and its density g/cm 3 is 6.15.The symbol for Iron is Fe and its density g/cm 3 is 7.87.The symbol for Iridium is Ir and its density g/cm 3 is 22.5.The symbol for Indium is In and its density g/cm 3 is 7.31.The symbol for Holmium is Ho and its density g/cm 3 is 8.80.The symbol for Hafnium is Hf and its density g/cm 3 is 13.3.The symbol for Gold is Au and its density g/cm 3 is 19.3.The symbol for Gallium is Ga and its density g/cm 3 is 5.91.
 The symbol for Gadolinium is Gd and its density g/cm 3 is 7.90. The symbol for Erbium is Er and its density g/cm 3 is 9.07. The symbol for Dysprosium is Dy and its density g/cm 3 is 8.55. The symbol for Copper is Cu and its density g/cm 3 is 8.96. The symbol for Cobalt is Co and its density g/cm 3 is 8.86. The symbol for Chromium is Cr and its density g/cm 3 is 7.15. The symbol for Cesium is Ac and its density g/cm 3 is 1.93. The symbol for Cerium is Ce and its density g/cm 3 is 6.77. The symbol for Calcium is Ca and its density g/cm 3 is 1.54. The symbol for Cadmium is Cd and its density g/cm 3 is 8.69. The symbol for Bismuth is Bi and its density g/cm 3 is 9.79. The symbol for Beryllium is Be and its density g/cm 3 is 1.85. The symbol for Barium is Ba and its density g/cm 3 is 3.62. The symbol for Antimony is Sb and its density g/cm 3 is 6.68. The symbol for Aluminum is Al and its density g/cm 3 is 2.70. The symbol for Actium is Ac and its density g/cm 3 is 10. There are 70 different metals charted in the periodic table. The defining difference between both categories of metals is that base metals oxidize easily while precious metals do not quite so easily. Metals are often divided into base metals and precious metals. This is known as electrical and thermal conductivity. Another characteristic of metals is that they can conduct electricity and heat. “Life should be like precious metals: weigh much in little bulk.” - SenecaĪlthough metals tend to be strong, tough, and ductile, it is also true that they are malleable. Metals are elements that are easily recognized by their physical properties of solidity and because they have what we logically think of as a “metallic” luster. To put it in simpler terms, the density of an element (metal or not) is the measure of mass per unit of volume.” It is important to note that all elements have density but in this article, we will just focus on the densities of metals, specifically. The mass of a substance per unit volume”, or “the distribution of a quantity (such as mass, electricity, or energy) per unit usually of space (such as length, area, or volume). So, what is the density of all metals? What Is Density?īefore we go into the specific density for each of the metals in the periodic table, we need to understand what density actually is when we are talking about chemistry.Īccording to the Merriam Webster dictionary online, density is defined as follows: There is a lot more information that scientists need to be au fait with. But just merely knowing the name of each and every one of the elements is not enough. Knowing the periodic table is key for most scientists. The density of metals ranges from Osmium at the highest density to lithium at the lowest density of any metal.
The symbol for Gadolinium is Gd and its density g/cm 3 is 7.90. The symbol for Erbium is Er and its density g/cm 3 is 9.07. The symbol for Dysprosium is Dy and its density g/cm 3 is 8.55. The symbol for Copper is Cu and its density g/cm 3 is 8.96. The symbol for Cobalt is Co and its density g/cm 3 is 8.86. The symbol for Chromium is Cr and its density g/cm 3 is 7.15. The symbol for Cesium is Ac and its density g/cm 3 is 1.93. The symbol for Cerium is Ce and its density g/cm 3 is 6.77. The symbol for Calcium is Ca and its density g/cm 3 is 1.54. The symbol for Cadmium is Cd and its density g/cm 3 is 8.69. The symbol for Bismuth is Bi and its density g/cm 3 is 9.79. The symbol for Beryllium is Be and its density g/cm 3 is 1.85. The symbol for Barium is Ba and its density g/cm 3 is 3.62. The symbol for Antimony is Sb and its density g/cm 3 is 6.68. The symbol for Aluminum is Al and its density g/cm 3 is 2.70. The symbol for Actium is Ac and its density g/cm 3 is 10. There are 70 different metals charted in the periodic table. The defining difference between both categories of metals is that base metals oxidize easily while precious metals do not quite so easily. Metals are often divided into base metals and precious metals. This is known as electrical and thermal conductivity. Another characteristic of metals is that they can conduct electricity and heat. “Life should be like precious metals: weigh much in little bulk.” - SenecaĪlthough metals tend to be strong, tough, and ductile, it is also true that they are malleable. Metals are elements that are easily recognized by their physical properties of solidity and because they have what we logically think of as a “metallic” luster. To put it in simpler terms, the density of an element (metal or not) is the measure of mass per unit of volume.” It is important to note that all elements have density but in this article, we will just focus on the densities of metals, specifically. The mass of a substance per unit volume”, or “the distribution of a quantity (such as mass, electricity, or energy) per unit usually of space (such as length, area, or volume). So, what is the density of all metals? What Is Density?īefore we go into the specific density for each of the metals in the periodic table, we need to understand what density actually is when we are talking about chemistry.Īccording to the Merriam Webster dictionary online, density is defined as follows: There is a lot more information that scientists need to be au fait with. But just merely knowing the name of each and every one of the elements is not enough. Knowing the periodic table is key for most scientists. The density of metals ranges from Osmium at the highest density to lithium at the lowest density of any metal.







 0 kommentar(er)
0 kommentar(er)
